Background: Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet counts and increased bleeding rate. Current first-line ITP treatments include corticosteroids and immunoglobulins. If first-line therapy fails, subsequent treatment options include the thrombopoietin receptor agonists (TPO-RAs). Avatrombopag (AVA), the most recently approved TPO-RA, is a small molecule, administered orally with no food-type restrictions, and no significant hepatotoxicity. AVA was approved by FDA in 2019 and EMA in 2021, for the treatment of chronic ITP in adult patients who are refractory to other treatments. The Phase 4 ADOPT study (NCT04943042) will generate data on the real-world effectiveness and safety of AVA in routine clinical practice in Europe. Described here are the interim baseline characteristics of patients enrolled in the ADOPT study.
Study objective and methods: ADOPT is a multicenter, observational study with the primary objective to describe the real-world effectiveness of AVA treatment over a prospective period of 12 months. Prospective data will be collected at routine clinical visits. In addition, retrospective data will be collected from patients' medical records for up to 12 months prior to AVA treatment start. Eligible patients must be ≥18 years of age, have provided informed consent, have an established and well documented ITP diagnosis, and are treated with, or initiating treatment with AVA for ITP, at enrollment. Exclusion criteria include enrollment in other clinical interventional studies, or intake of an investigational medicinal product, ≤3 months prior to inclusion.
The primary endpoint is the cumulative number of weeks with a platelet count ≥30×10 9/L. Platelet counts during rescue medication use and within 4 weeks after stopping a rescue medication or following splenectomy are considered non-response and thus not included in the primary endpoint cumulative number. Secondary endpoints supporting the primary objective include cumulative number of weeks with a platelet count ≥50×10 9/L, patients (n [%]) with a platelet count ≥30×10 9/L and ≥50×10 9/L for at least 8 consecutive weeks, patients (n [%]) experiencing WHO bleeding grade ≥2, patients (n [%]) requiring rescue medication, and time from AVA treatment start to platelet count ≥30×10 9/L and ≥50×10 9/L. Additional secondary endpoints are AVA dose and dosing frequency, reason for ITP treatment discontinuation or change from one ITP treatment to another (prior to and during the study), use of concomitant ITP medications, physicians' satisfaction with outcome of AVA treatment (5-point scale), physician assessment of clinically meaningful effects (Clinical Global Impression of Change scale), healthcare resource use, as well as a number of patient reported outcomes (PROs). Secondary safety endpoints include serious adverse events and adverse events of special interest (thromboembolic events [TEEs] and significant bleeding events).
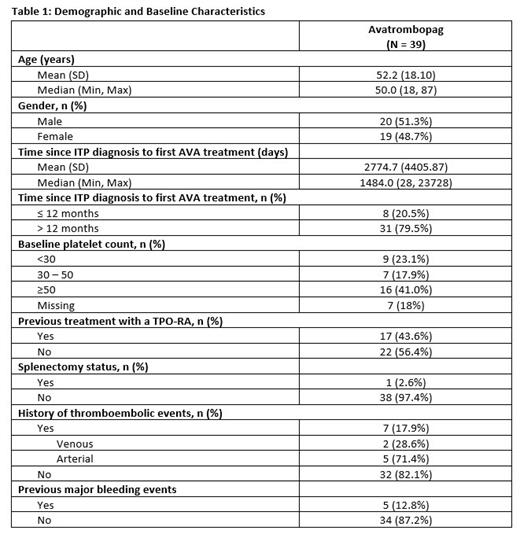
Results: Fifty-six patients have been enrolled in the study as of July 2023, and demographic and baseline characteristics information were available for 39 patients (Table 1). The mean age (SD) was 52.2 (18.10) years and 20 patients were male (51.3%). Nine patients had a baseline PC <30x10 9/L. Eight patients (20.5%) reported newly diagnosed/persistent ITP with <12 months disease duration since diagnosis; 31 (79.5%) had chronic ITP. A majority of patients (n=22; 56.4%) had no previous exposure to TPO-RAs. One patient was splenectomized (2.6%), and seven had experienced prior TEEs (17.9%).
Conclusions: This baseline interim data set from the ADOPT Phase 4 study provides important insight into the types of ITP patients who are being prescribed avatrombopag across European centers. More than half of enrolled patients were TPO-RA naïve, and over 20% did not have chronic ITP although the mean duration of ITP was 7.6 years and median duration of 4 years at the time of first AVA treatment. Though previous clinical trials excluded patients with newly diagnosed/persistent ITP and prior TEEs, this real-world evidence study does not exclude such patients. We look forward to presenting safety and efficacy data, as well as multiple PROs, from this observational study at future scientific meetings.
Study status: The study was initiated in January 2022, will include approximately 60 sites in Europe, and enroll 150 patients in total.
Disclosures
Ghanima:Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Sobi, Pfizer: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Bayer: Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria; UCB: Consultancy, Honoraria; Kedrion: Consultancy; cellphire: Consultancy, Honoraria; alpine: Consultancy, Honoraria; hibio: Consultancy, Honoraria; BMS: Honoraria, Research Funding. McDonald:Rigel: Research Funding; Amgen: Consultancy; Novartis: Consultancy; Sobi: Consultancy; Grifols: Research Funding. Putnik:Swedish Orphan Biovitrum AB: Current Employment. Jamieson:Sobi, Inc.: Current Employment. Lethagen:Swedish Orphan Biovitrum AB: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal